Webinar on Ex vivo MRI sample preparation : Reports
Report on the SAIN discussion on setting up ex vivo acquisitions and more specifically on the use of PFPE fluorinated products
Background:
The ex vivo analysis of (biological) samples by MRI is very often hampered by the useless signal coming from the solution in which the sample is immersed, but without this solution we come up against significant field distortion due to air-sample interface susceptibility problems.
Discussion
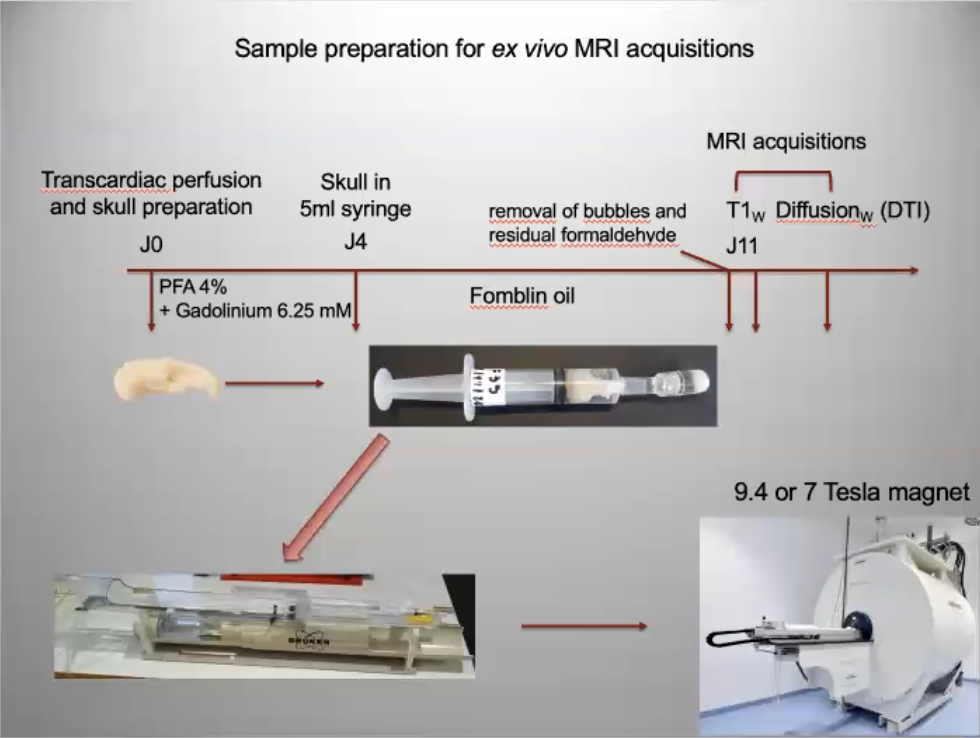
To overcome this problem, many sites use PFPE-type fluorinated products. As these liquids consist solely of carbon and fluorine nuclei, they are invisible in proton MRI/NMR, and their magnetic susceptibility is close to that of water, enabling them to greatly limit field discontinuity problems. These compounds have the advantage of being chemically inert and non-toxic, but they are classified as ozone-depleting compounds, which explains why a certain number of them are no longer produced, or are in the process of being phased out. The main PFPEs used on our various platforms are : FOMBLIN® Y06 from solvay™ (price per kg ?) GALDEN® HT70 from Solvay™ (<300€/kg) FLUORINERT® FC40 or FC70 3M™ (price per kg?) NOVEC® 7300 3M™ (price per kg?) CF2 THERMASOLV™ (price per kg?)
These oily compounds have a density ranging from 1.5 to 2 times that of water, so the biological sample tends to float and should be cushioned, for example, with degassed foams. These compounds are not soluble in either water or alcohol, and must therefore be “rinsed” manually by gentle swabbing and/or squirting. As PFPEs are expensive, it is quite possible to recover the liquid, filter it through a micro-pore filter and reuse it by storing it in another bottle.
If the sample has been doped with gadolinium and fixed in formaldehyde, its T1/T2 properties will change over time, but more by passive diffusion of the gadolinium than by any property of the PFPE. Note: if the initial perfusion has been carried out correctly (notion of retrograde perfusion), contrast changes little over time. => after 7 days of maceration in the gadolinium-doped medium, the contrast of the sample stabilizes (time for the gadolinium to penetrate homogeneously into the tissues) => similarly, if a doped sample is macerated in PFPE for too long, the contrasts will fade (diffusion of Gadolinium into the PFPE). => much of the gadolinium can be removed by passive diffusion by leaving the sample to macerate for two weeks in undoped PBS. => After 3/4 years, contrast degradation is probably caused by formaldehyde, so if the sample is stored in formalin, it may be worth rehydrating it in PBS before handling.
Starch ?
A very interesting point discussed briefly was replacing PFPE with potato starch. https://www.sciencedirect.com/science/article/pii/S2589004222019678 The T2 of suspended water drops very sharply, making it invisible to standard sequences. This is a very interesting point to investigate because :
the cost of starch is negligible the susceptibility of the medium can be considered to form a continuum with that of the sample starch rinsing is trivial the starch solution may have been doped with gadolinium to the same molarity as the sample, without inducing passive extraction of the gadolinium present in the sample and without giving a signal. The density of the medium allows better degassing of microbubbles The sample no longer floats The rheopectic properties of starch (increased viscosity under shear strain) mean that it absorbs the vibrations generated by the gradients.